Five Key Points You Need to Know About Ethylene Oxide (EtO) Safety
The attention on Ethylene Oxide safety and USEPA’s recently proposed rules has raised the need among various stakeholders for reliable information in support of effective management of EtO.
Interest in potential hazards of EtO has increased in recent years in large part because of the U.S. Environmental Protection Agency’s (USEPA) reassessment of EtO’s toxicity and its April 2023 proposals for handling and emissions of EtO.
The aim of this post is to provide producers, users, regulators, communities, and others with information that contributes to their views on management of EtO and public health. We focus on existing knowledge about the ways that people can be exposed to EtO and the magnitude of those exposures.
Background
In the 1980s, research on EtO exposure and toxicity led federal agencies to introduce workplace and environmental regulations designed to protect employees and the public. Two of the controlling regulations are 29 CFR 1910.1047 issued by the Occupational Safety and Health Administration (OSHA) and 40 CFR 63 Subpart O issued by USEPA. Producers and users of EtO duly incorporated the federal rules into their routine operations for occupational health, permitting, waste disposal, recordkeeping, and reporting.
Meanwhile, new research appeared in the scientific literature that reported on studies of cancer incidence among people exposed to EtO while at work. A series of publications from a longitudinal cohort study of sterilization facility workers initiated by the National Institute of Occupational Safety and Health (NIOSH) received particular attention.
The results of those and similar studies shaped programmatic analyses and regulatory processes for ethylene oxide safety at USEPA beginning in approximately 2006. Those activities led to a determination by USEPA in 2016 that EtO is a more potent carcinogen than it previously understood. Following that finding, the Agency updated its human health risk assessment for EtO in 2020, announced its intention to update its requirements for management and emissions of EtO, and held public meetings in 25 communities around the country.
In April 2023, USEPA released proposals that, if enacted, would require manufacturers and users to lower emissions and increase reporting on EtO. See a prior post for a summary of those proposals and near-term suggestions for organizations in the healthcare sector that use EtO.
The attention on Ethylene Oxide safety and USEPA’s recently proposed rules has raised the need among various stakeholders for reliable information in support of effective management of EtO:
- Producers and users of EtO need to understand their regulatory compliance requirements, the proposed updates on handling and disposal of EtO, the best available science on EtO toxicity, and the performance of their workplace and environmental controls.
- Regulators need to understand the sources and pathways of total human exposure to EtO and the policy options for protection of workers and communities.
- Communities need reliable information that supports informed perspectives on the benefits and risks to public health of EtO.
Addressing the entirety of those needs is beyond the scope of a single post, but here we provide the foundational information on EtO that is necessary to understand the public health issues around EtO. We begin with information on the properties of EtO that influence its occurrence in the environment, then move on to sources of EtO, and close with pathways of exposure to EtO.
Physical and Chemical Properties
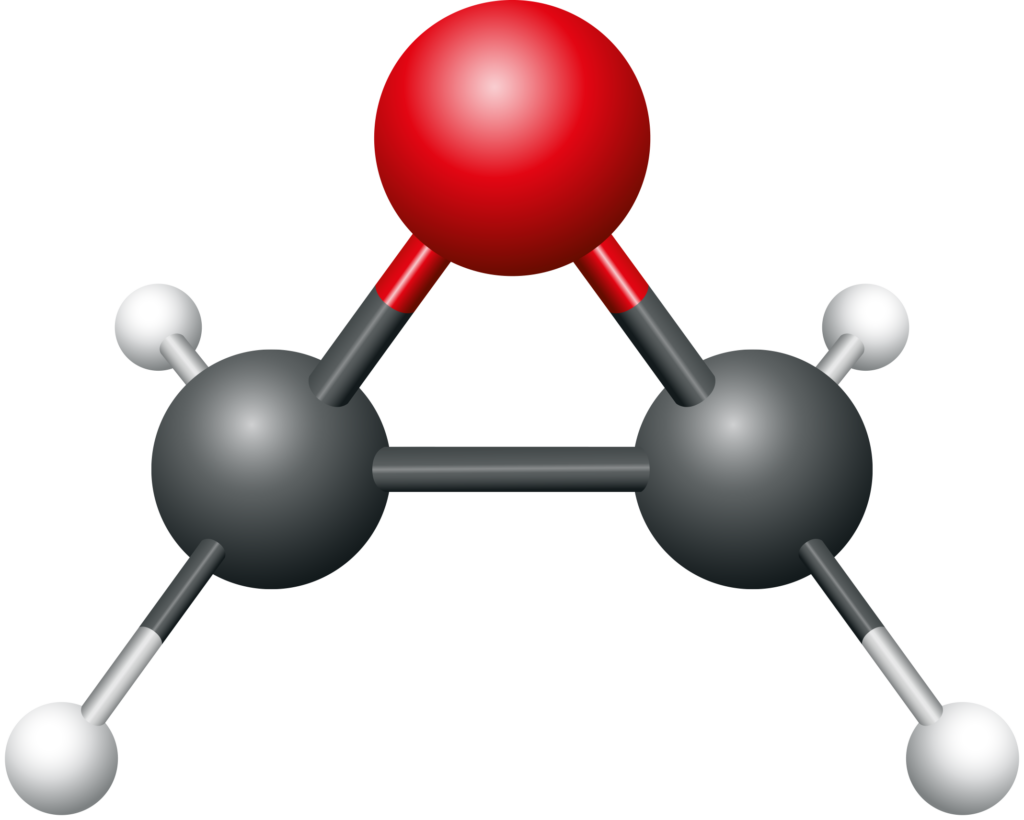
Ethylene oxide consists of two carbon atoms, four hydrogen atoms, and one oxygen atom and its chemical formula is C2H4O. A distinguishing characteristic of EtO is the three-sided ring of carbon (grey) and oxygen (red) atoms shown in the figure below. The nature of the ring makes it easy for EtO to react with other substances and contributes to its ability to sterilize medical devices.
As with many other relatively small molecules primarily composed of carbon and hydrogen, EtO is a gas at ordinary environmental temperatures and pressures. Therefore, most emissions of EtO are to the atmosphere as opposed to water bodies, soil, or landfills. EtO gas is colorless and odorless, except at very high concentrations where it has a sweet smell, which means the presence of EtO is primarily limited to specialized measurement methods.
Although a gas in normal environmental settings, EtO also mixes easily with water, largely because of the oxygen atom in its structure. Its water solubility has some practical applications and implications. For instance, a category of air pollution control systems commonly known as scrubbers work by spraying water droplets onto an EtO-containing gas stream. The water droplets intercept molecules of EtO and carry them down to a reservoir at the base of the scrubber. A similar phenomenon occurs in the environment where rainfall can wash EtO from the atmosphere.
Sources
Chemical manufacturers in the U.S. produce approximately 6,400 million pounds of EtO each year. Most of the EtO is used to manufacture other chemicals, such as ethylene glycol. Much of the rest is used to sterilize agricultural products and medical devices and supplies.
According to EPA, commercial sources released 135,088 pounds of EtO to the atmosphere in 2020. That amount represents less than one-tenth of one percent of the EtO produced that year. Estimates show chemical manufacturers account for nearly three-quarters of EtO emissions, while facilities that sterilize medical equipment and supplies or agricultural products account for about one-fifth.
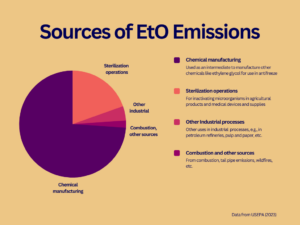
In addition to emissions from commercial operations, EtO is thought to be a byproduct of combustion. However, USEPA has stated that its current understanding of emissions from combustion is inadequate and plans to test for EtO in tailpipe emissions from motor vehicles.
EtO is also produced inside the human body as a byproduct of normal metabolism. This topic is discussed in later in this post.
Hazards
Flammability and reactivity are two of the primary hazards of EtO. It can ignite at concentrations in air above 3%. Therefore, producers and users of concentrated EtO avoid conditions that present a risk of ignition. To do so, they maintain concentrated EtO in sealed piping and vessels, only allow use of non-sparking tools in areas where concentrated EtO could be present, and take many other precautions. While only a few industrial accidents have been reported, at least three people have died and others have been injured in explosions, underscoring the importance of safe chemical management.
EtO can also be toxic to humans and other organisms. EtO is widely considered to be a carcinogen because of its mutagenicity. For example, USEPA, the US Department of Health and Human Services, and the International Agency for Research on Cancer all identify EtO as a carcinogen. EtO has also been linked to reproductive and selected other effects.
Exposures
The major routes of human exposure to EtO are thought to be: (1) metabolism and (2) inhalation. While low levels of EtO have been reported in certain foods, intake via ingestion is likely negligible.
EtO exposure can be assessed directly by measuring amounts of compounds formed when EtO binds with DNA or hemoglobin. These compounds are called adducts. The primary adducts specific to EtO are N7-HEG [also known as N7-(2-hydroxyethyl)guanine] and HEV [also known as 2-hydroxyethylvaline].
The US Centers for Disease Control and Prevention (CDC) routinely measures EtO adducts in the general population. CDC’s surveys show that essentially everyone in the entire U.S. population has measurable levels of HEV adducts.
Like other biomarkers, these adducts reflect total intake of EtO but do not provide information on the pathways of exposure. To understand the pathways, we must appeal to exposure assessments and environmental monitoring described in the scientific literature. We begin with internal sources of EtO exposure.
Endogenous Production
Humans produce EtO inside their bodies, a source that is referred to as “endogenous EtO”. This EtO is formed as a byproduct of metabolism of ethylene, which is also produced by the body. Clearly, this source of EtO exposure is unavoidable. The amount of endogenous EtO generated in the body has been estimated to be equivalent to inhaling 2 to 3 parts per billion by volume [ppb(v)] of airborne EtO. As discussed next, that concentration is about 10-fold higher than average EtO levels in outdoor air.
Outdoor Air
Research shows that EtO is present in outdoor air across the United States. Testing by USEPA at 51 locations around the country in 2021 yielded an average EtO concentration of approximately 0.2 ppb(v). Earlier monitoring data from 18 sites in nine states during 2018 and 2019 produced similar findings. USEPA has reported that some of those measurements could be overestimated because of possible interference related to the sampling method. Nonetheless, because of its ubiquity, inhalation of EtO in outdoor air likely occurs continuously for most people.
Smoking
Smokers have substantially elevated EtO exposure compared to non-smokers. HEV adducts clearly demonstrate the effect of smoking. According to data reported by the CDC, the average concentration in active smokers is approximately 200 picomoles of HEV per gram (pmol/g) of hemoglobin compared to approximately 20 pmol/g in non-smokers. Non-smokers who live or work in buildings where others smoke indoors may have elevated exposures to EtO as well.
Workplaces
Some workers experience elevated exposure to EtO compared to the general population. This group includes people who work in areas where EtO is manufactured or used to sterilize products. The amount of published data on current workplace exposure concentrations is limited, but USEPA recently reported average exposure concentrations of 230 ppb(v) for commercial sterilization employees and 120 ppb(v) for healthcare employees that work with EtO.
Facility Emissions
Facilities that manufacture EtO or sterilize with EtO release residual amounts of the gas to outdoor air. Those emissions have the potential to contribute to EtO concentrations in areas nearby.
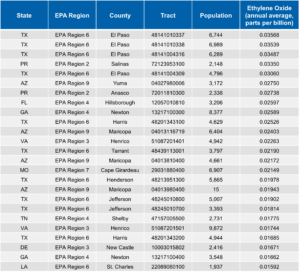
As part of its AirToxScreen program, USEPA periodically evaluates the possible impact of those emissions on air quality. The Agency combines data on facility emissions, meteorology, and local populations to estimate the annual average EtO concentration at the center of each census tract in the country.
USEPA’s most recent analysis is based on emission rates reported by organizations for calendar year 2018. The table below shows the 25 census tracts with the highest modeled EtO concentrations according to that analysis. The EtO concentrations range from 0.018 ppb(v) to 0.030 ppb(v). All of these modeled concentrations are less than 0.2 ppb(v), the average EtO concentration in outdoor air reported by USEPA.
Health Risks
A widely applied science policy convention in public health is that a linear relationship exists between the cumulative dose of a chemical carcinogen and the probability a tumor will form in a person. Cumulative dose is the total amount of a chemical inhaled or ingested over a period, such as the amount of EtO inhaled over a lifetime. Generally speaking, the higher the long-term average exposure concentration holding duration constant or the longer the duration of exposure holding exposure concentration constant, the larger the cumulative dose.
Research published in the scientific literature is helpful for understanding potential cumulative doses of EtO. For example, the cumulative dose from endogenous EtO likely substantially exceeds that from pathways of EtO exposure because the concentrations are relatively high and the exposure is continuous. Also, measurements and modeling by USEPA indicate that background levels of EtO in outdoor air are likely to be a continuous source of EtO exposure as well, but at lower concentration than endogenous EtO. In addition to contributions from within the body and outdoor air, people who work with EtO or smoke over many years likely have cumulative exposures that are greater than those for the general population. Finally, modeling by USEPA indicates that cumulative exposures directly related to emissions from facilities that produce or use EtO appear to be relatively low for the general population compared to other sources of exposure.
Five Key Points About Ethylene Oxide Safety
- Ethylene oxide is manufactured for use in numerous important commercial applications.
- Facilities involved in chemical production and medical device sterilization are the principal recognized sources of EtO, while emission from other sources, such as combustion of fossil fuels or biomass, are less well understood.
- Flammability, reactivity, and toxicity, including carcinogenicity, at elevated concentrations are the most notable hazards of EtO.
- Human exposure to EtO occurs through several different pathways, such as production within the human body; background levels in outdoor air; tobacco smoking; certain workplace environments; and emissions from commercial operations.
- For a typical person in the US, cumulative exposure to EtO appears to be driven largely by background levels in outdoor air and endogenous production, although smoking and working with EtO has been shown to often result in exposures above those for the general public.
Subscribe
to our blog
"*" indicates required fields





